Platform24’s triaging software achieves EU MDR certification
Ahead of the curve during the most significant overhaul in European regulation for medical devices, Platform24 is excited to announce Triage24 as the first Class IIa MDR-certified triaging software on the market.* Undergoing a thorough and rigorous application and auditing process, the software’s innovative and central triaging module has proven itself an exceptional product upholding the highest standards of safety for our partners and their patients.
Triage24: MDR certified as of 28th of June, 2022
Triage24 is an advanced symptom checker and triaging software module that helps patients get the care they need, when and where they need it. Developed by an expert team of doctors and engineers, our module stands out from other off-the-shelf symptom checkers by deeply integrating with region-wide healthcare and IT-systems (e.g., EHRs) to offer medically safe, individualized recommendations based on the healthcare services available. In other words, it matches patient demand with the current healthcare supply, saving all stakeholders time and resources and improving health outcomes.
Changing Regulation: An obstacle or opportunity?
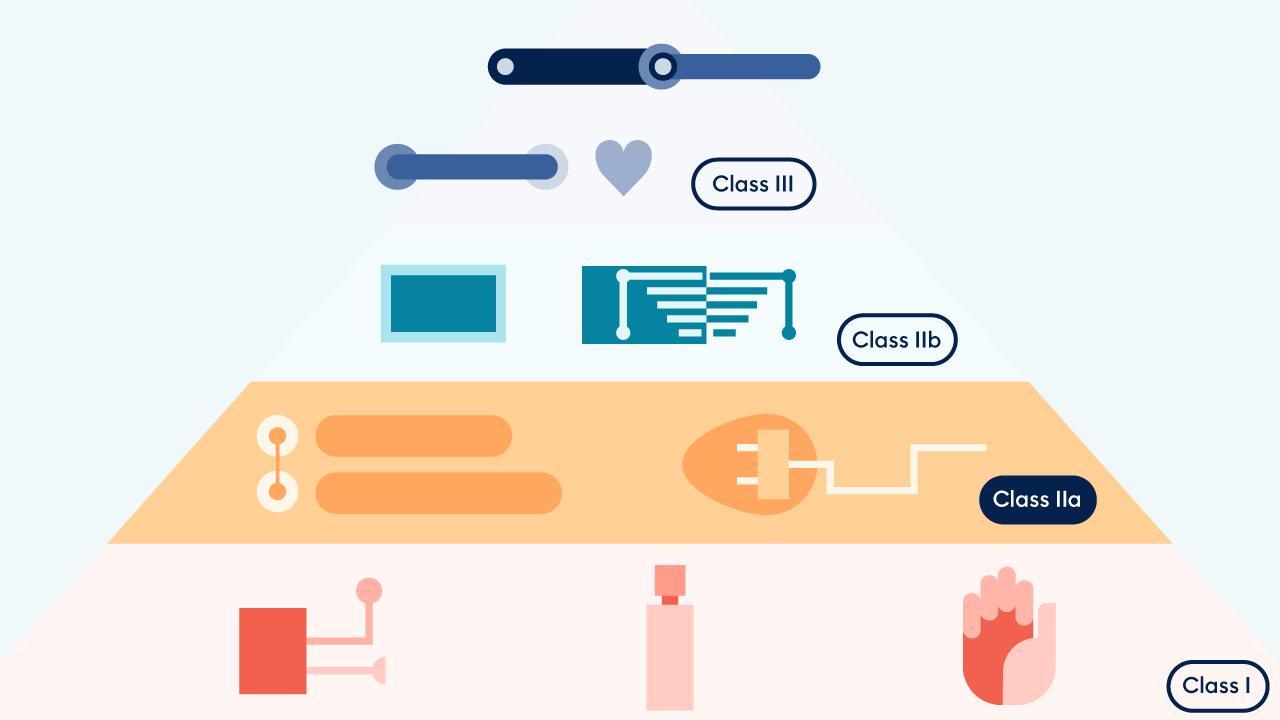
For some companies, the current phase-out of the MDD certificate and transition to MDR certification poses an insurmountable obstacle. Indeed, experts predict that 10% of companies and 30% of products could disappear from the market due to increased restrictions during the current transition.
Platform24, however, presents a unique opportunity to utilize the data from real-world evidence already being collected and to obtain a more apt risk classification. Drawing on more than 450,000 patient interactions per month and 230 implementations, Triage24 proved its efficacy and safety. The result: Triage24 is now a Class IIa, MDR-certified medical device, and its availability across EU markets will continue uninterrupted after the MDD expiration period.
MDD to MDR: What changed?
In 2017, the Medical Device Regulation (MDR) certification was established, revising the previous Medical Device Directive (MDD) process, to offer a more “robust, transparent, predictable and sustainable regulatory framework,” and to address constantly evolving safety requirements for medical devices, including software. This new, European-specific regulation not only increased evaluation and monitoring standards but also set forth procedures to re-classify MDSW (medical device software).
The switch from MDD to MDR is a difficult and lengthy process. It involves stricter requirements for transparency (e.g., technical documentation and audits), traceability, and clinical evaluation, including post-market assessments and investigations. In addition, there are heightened demands on cybersecurity elements, usability, and expertise of responsible persons.
Paperwork aside, these stricter standards neatly align with the Platform24 mission to create safe, seamless and streamlined healthcare journeys, and the higher expectations for usability and clinical evidence are key.
Wider usability through a single-entry point interface
MDR requires a higher level of “user-friendliness” in order to minimize health risks due to user misuse. Platform24 develops solutions that work not just for nisched groups, but for every practitioner and patient in a healthcare system. Our single entry-point creates seamless workflows for all users – staff, clinicians, and patients – not just the self-selecting “digitally savvy.” All modules are channeled through the platform’s digital front door to streamline the user experience so that the focus is on patient care rather than technology.
Proven safety through a data-driven approach
Our platform is designed to thrive on data, collecting both real-world and clinical information. This data-driven approach helps track, monitor, analyse and improve Triage24’s triaging logic. It also produces a significantly higher amount and quality of clinical evidence to meet MDR control and vigilance requirements.
To explain this improved approach, let’s first examine a traditional triage situation: a patient calls a health advice hotline, and the advice nurse offers a recommendation. The process ends there. In this situation, it is difficult to validate the safety and efficacy of this service, and outcomes are not closely monitored. In other words, it lacks the clinical evidence to verify if the patient even received needed care and to assess the quality of the advice.
Triage24, on the other hand, follows the patient journey, collecting structured data to validate whether the logic is effective and safe. A truly enterprise solution, this module goes further, effortlessly adapting to local circumstances and currently available care services in real time, which helps our partners better utilize resources and helps patients find care when and where they need it.
Upcoming Certifications – You24
Growing our offerings for MDR certified solutions, You24 is also undergoing the evaluation process. This solution helps our partners enhance chronic care journeys by integrating and acting on data from remote monitoring.
Stay tuned for more announcements by subscribing to our newsletter…
*Based on internal market research
Written in collaboration with Amy Harris
Jingcheng Zhao MD PhD
Research and Real-World Data Manager
A physician-scientist dedicated to producing clinically and policy-relevant evidence using real world data.